Abstract
Disruption of TP53 gene occur in a subset of patients with refractory/relapse B-cell non-Hodgkin lymphoma (r/r B-NHL) and confer inferior prognosis. Recently, we reported the safety and efficacy of two clinical trials, administrating CAR T-cell infusion either alone (Trail A) or incorporated in ASCT (Trial B), in the treatment of r/r B-NHL. To address the prognostic impact of TP53 alterations on r/r aggressive B-cell lymphoma when treated with CAR T-cell therapy, in the present study, we systemically evaluate the therapeutic effects among the patients with TP53 alterations in these 2 trials with expanded cohort size and extended follow-up.
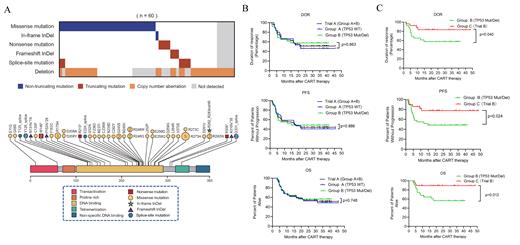
In two trials, we enrolled 34 patients in group A (TP53wt in trial A), 32 patients in group B (TP53 alteration in trial A) and 28 patients in group C (TP53 alteration in trial B). Baseline features of patients in three groups were balanced. Heterozygous mutations and/or deletions of TP53 gene detected in 60 patients. A total of 42 (70.0%) patients carried either TP53 mutation(s) or sole del(17p), including 27 (45.0%) harboring TP53 mutation(s) and 15 (25.0%) carrying TP53 deletions. Biallelic TP53 aberration was revealed in the remaining 18 (30.0%) patients. They harbored TP53 mutation also carried del(17p) in the second allele. Among the 51 mutations that identified (Figure 1A), 37 were missense mutations, 6 were splice-site mutations, 4 were nonsense mutations, 3 were frameshift insertions or deletions (indels), and 1 was non-frameshift indel. Forty-five mutations (88.0%) located in DNA-binding domain (DBD, amino acid 101 - 294). Totally, 40 (66.7%) patients carried loss-of-function aberrations that resulted in TP53 inactivation, including either copy number loss or truncated mutations.
Of the patients harboring TP53 alterations (group B), with a median follow-up of 16.7 months (range: 3.1 to 41.0 months), the median DOR and OS was not reached. The median PFS was 14.8 months (95% CI: 5.1 - NE). The estimated 24-month PFS and OS rates were 48.4% (95% CI: 30.2% - 64.4%) and 56.3% (95% CI: 36.6% - 72.0%). Notably, the DoR, PFS and OS were similar between the patients with (group B) or without (group A) TP53 alterations when treated with CAR19/22 T-cell cocktail therapy (Figure 1B). Since prognostic relevance varied according to distinct TP53 alterations, four different functional classification systems were applied to further elucidate whether CAR T-cell cocktail therapy can overcome the unfavorable prognostic impact conferred from TP53 alterations. Remarkably, in each classification system, either best ORR or survivals did not differ significantly between patients stratified in distinct risk subgroups, indicating CAR19/22 T-cell cocktail therapy could overcome the negative impact of TP53 alterations in these patients.
Among the 28 patients (group C in trial B) who had TP53 alterations and were treated with ASCT incorporating CAR19/22 T-cell cocktail, 26 patients (92.9%, 95% CI: 77.4% - 98.7%) achieved best ORR, with 23 (82.1%, 95% CI: 64.4% - 92.1%) having a CR. With a median follow-up of 21.2 (range: 4.0 - 48.7) months, the median DOR, PFS and OS were not reached. The estimated 24-month PFS and OS rates were 77.5% (95% CI: 56.5% to 89.3%) and 89.3% (95% CI: 70.4% - 96.4%), respectively. At data cutoff date, 22 patients (78.6%, 95% CI: 60.5% - 89.8%) maintained their initial responses while 6 patients (21.4%, 95% CI: 10.2% - 39.5%) experienced disease progression. Strikingly, among the patients with TP53 alterations, as compared with CAR19/22 T-cell cocktail therapy (Group B), significantly extended DOR (P = .040), PFS (P = .024) and OS (P = .012) were achieved when CAR T-cell cocktail infusion was incorporated in ASCT (Figure 1C).
In conclusion, our study, at first time, has proved that CAR T-cell cocktail therapy is effective for TP53-disrupted r/r aggressive B-NHL. Incorporating CAR-T cell infusion in ASCT can further improve the long-term outcome of these patients. Future clinical trials need to be performed with larger cohort to get more definitive conclusion that if CAR T-cell therapy should be moved earlier for r/r B-NHL in the coming era of immunotherapy.
No relevant conflicts of interest to declare.